Overcoming Challenges in RTM Device Reimbursement with IoT
By Carsten Brockmann
November 19, 2024
By Carsten Brockmann
November 19, 2024
Estimated reading time: 11 minutes
One of the fastest-growing medical technology (medtech) segments is remote therapeutic monitoring (RTM). RTM includes Internet of Things (IoT) devices, such as wearables, implants and mobile apps.
These devices capture real-time data and monitor a patient’s health condition and progress. This enables health care providers to adjust treatment as needed. RTM IoT devices are revolutionizing health care and improving patient outcomes cost-effectively.

Before, patients had difficulty sharing this data, and health professionals faced obstacles in obtaining and billing for it. RTM with reimbursement codes makes all this possible and easier to track and manage.
As telehealth and the demand for medtech devices grow, reimbursement policies are being updated and expanding. RTM reimbursement must be optimized and approved for new devices as they come to market to manage health issues like chronic disease better. Otherwise, delays could stunt progress in patient care and the device market.
The right IoT connectivity technology must be reliable and secure. It must also be easy to manage regardless of where the patient is. Patients and health care professionals rely on always-on connectivity. It’s vital to maintain the requirements for reimbursement.
The adoption of new and more affordable medtech devices is driving the medical device reimbursement market. By 2027, it’s predicted that the global medical device reimbursement market will increase at a compound annual growth rate (CAGR) of 8.4%. It will reach a market value of $602.9 billion.
Three key trends are fueling the adoption of innovative medtech devices.

They include:
The government is heavily advocating for digital health care and RTM. Remote health care has proven to manage chronic diseases better, reduce costs and make health care more accessible.
Any massive growth comes with challenges. This includes approvals for reimbursement for medtech devices like RTM.
Medical device reimbursement is a complex process in which insurance companies pay health care providers for prescribed devices and services.
There are many rules and restrictions on services and billing. Innovators must stay informed about expanding coverage and code changes to maximize potential reimbursement opportunities. Without proper reimbursement, a medical device would become too costly for providers.

Centers for Medicare & Medicaid Services (CMS) finalized telehealth service policies, including RTM for 2024. Here are a few key areas to note:
The biggest win for digital health companies, providers and patients is stand-alone reimbursement for RTM services. These are provided by federally qualified health centers (FQHCs) and rural health clinics (RHCs). The proposed rule is also open to feedback from stakeholders on these policies.
On the other hand, some things must be resolved. A generic RTM device billing code was not approved. CMS still had concerns about assigning a reimbursement value for devices with a wide range of costs and benefits.
The 2024 final rule is a start in making progress. Unfortunately, there are several areas of uncertainty in the codes, such as:
In addition, there are different codes for services and products that make things even more complex.
Current Procedural Terminology (CPT®) service codes identify initial setup and patient education on the devices. There are codes for medication adherence and therapy response. Interactive communications and activity over a certain period are also tracked to ensure patients stick to their therapy plan.
If the initial therapy study is positive, the Healthcare Common Procedure Coding System (HCPCS) reimburses for devices, supplies and services not covered by CPT codes. CMS is covering RTM costs for chronic conditions from sleep apnea to diabetes. The next section explains some of these use cases.

RTM is a proactive approach to health care services and chronic disease management that improves a patient’s quality of life. Patients can self-report the data to their providers or automatically generate it by connected medical devices.
Practitioners can track patients’ use and response to as-needed medications like insulin. This RTM data allows them to determine the correct therapy regimen.
Several use cases for connected health care leverage RTM. The following three use cases range from legacy to cutting-edge.
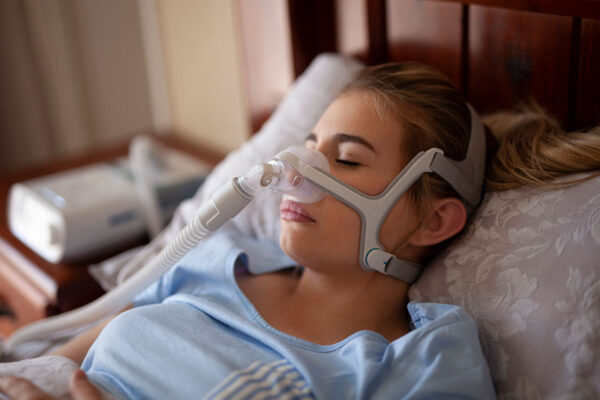
Doctors have used continuous positive airway pressure (CPAP) devices to treat sleep apnea for decades. RTM takes this a step further by measuring therapy adherence and treatment response.
In 2024, health care reimbursement in the U.S. has evolved to embrace the proven benefits of digital RTM, particularly for conditions like sleep apnea. Specific CPT and HCPCS codes streamline reimbursement for RTM, enabling financial coverage for patients and providers. Continuous monitoring and timely emergency intervention improve patient care and outcomes.
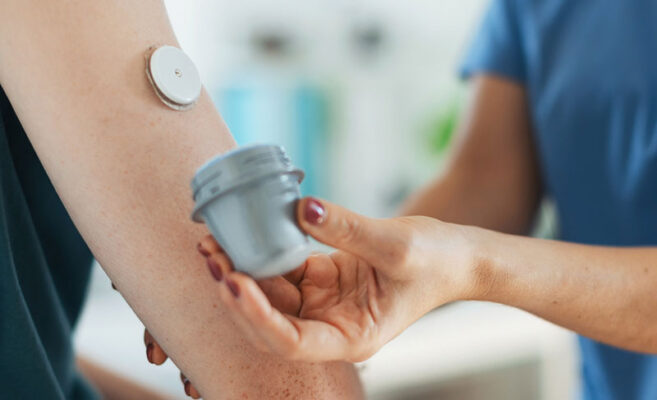
Medicare and Medicaid have expanded coverage for continuous glucose monitoring (CGM) sensors and insulin pumps, vital for diabetes management. Patients with diabetes treated with insulin or who have a history of hypoglycemia may be eligible for CGM devices.
Qualified practitioners and physicians must conduct six-month evaluations of diabetes control before prescribing CGM. Evaluations can be done through telehealth visits. RTM is a key component for reimbursement. It ensures patients receive the necessary care and support for their diabetes treatment plan.

Chronic pain costs the United States $635 billion per year while leaving patients disabled. For decades, virtual reality (VR) therapy has been studied and used to treat various acute and chronic pain conditions. Only recently did immersive VR headset technology finally become accessible and easy to use. Combined with cognitive behavioral therapy (CBT) techniques, immersive VR therapy is even more effective.
HCPCS reimbursement codes recognize VR therapies when monitored remotely. Immersive home-based VR therapies offer pharmacological options for pain management. These could transform chronic pain management.
There is unlimited potential for medtech devices to improve patient outcomes while reducing medical costs.
The 2024 HCPS coding for RTM creates new opportunities for patients and health care professionals. It also unlocks new revenue streams for medical device manufacturers.
RTM devices eligible for reimbursement are financially viable for providers and patients. This opens the doors to more device sales potential for manufacturers.
Reimbursement incentivizes health care providers to integrate RTM devices into their practices. More providers lead to wider market adoption and more demand for these devices.
Greater adoption can drive partnerships between health care providers and device manufacturers to improve reimbursement outcomes. Collaboration on new and improved devices can build relationships for other sales opportunities.
Despite the demand for RTM and new opportunities, there are challenges.

Approval of medtech devices goes through stringent regulatory requirements. Devices must be approved by the FDA and used as intended. Unfortunately, there is a lack of standards for policies across different regions.
A University of Chicago study examined the implications of Medicare’s outdated reimbursement policies for new medical devices. They found that delaying Medicare coverage for new medical devices between 2010 and 2022 saved a little money upfront. Nevertheless, the negative effects on patients were 11 times more significant in delaying health improvements.
In addition to affecting patient health, outdated policies increase health care costs. Coverage delays for new FDA-approved medical devices cost between $1.90 million and $2.80 million per year.
Outdated reimbursement policies and coverage delays are all missed opportunities to improve patient health and decrease costs to the U.S. Cuts in reimbursements affect the profitability of medical device companies.

Protecting patient data and privacy is a key concern. Health care providers can build trust by working with patients to safeguard their IoT data and devices from internal and external threats. Threats to data security can include vulnerabilities in connections, modules or platforms from countries with less strict privacy regulations.
To adhere to patient data privacy policies, solution providers typically roll out cybersecure and cloud-connected medical devices. These devices communicate via cellular modules and mobile connectivity plans to medical health clouds and electronic health record (EHR) systems. Unlike smartphone apps, data encryption and end-to-end authentication are standard components of cellular devices.
To make RTM or any medtech device work, you must be able to connect the provider’s and patient’s devices. Reliable, always-on mobile internet connections are vital to the health care ecosystem, including meeting the requirements for reimbursement.
Due to regulation limits and uncertain rates, providers need more flexibility in using RTM devices in their practices and to be properly reimbursed.
These costs are especially challenging for health care providers who must purchase a fleet of medtech devices for initial trials.
This can include completing certifications, managing multiple mobile operators and hardware providers, and maintaining contracts.
Adapting to different business models complicates deploying and managing connected devices and outcomes.
This is where NExT connected modules make life easier. They give providers and medtech companies more control and flexibility while lowering operational costs.

NExT connected modules, powered by Telit Cinterion, are the next-generation IoT as a service solution to optimize RTM. These all-in-one packages include cellular modules, connectivity and management tools.
NExT connected modules are delivered as a service. They enable real-time 24/7 monitoring for health care IoT applications regardless of patient location. A connectivity by design approach guarantees a reliable connection for your RTM devices, aligning with strict reimbursement codes.
Investing in connectivity hardware upfront results in long-term capital expenditures (CapEx). Telit Cinterion uses an operating expenses (OpEx) model. Medical device makers benefit from a monthly subscription model for cellular communication hardware and connectivity starting at $0.89 per month.
Hardware is part of the service offer. A monthly base fee is only required during the initial reimbursement phase. This enables easier entry into the market with less risk. After that, CapEx for the communication hardware is covered.
Patients can continue using the NExT connected module service and choose a data plan. They can use multi-IMSI for automatic network selection or an eUICC switch for manual control. This flexibility ensures continued device functionality for years to come.
Telit Cinterion offers an end-to-end solution that includes support to avoid delays and confusion and get to market quicker.
One unique platform bundles everything to manage the device, module and connectivity. The packages blend technology, coverage, management tools and business-friendly OpEx commercial terms.
This complete solution simplifies technical setup and business management. It combines hardware, connectivity and services into one easy-to-understand contract and bill at a cost-effective price.
NExT connected modules enable businesses to invest in growth instead of inventory. They can focus on aligning their RTM devices to the reimbursement codes for more adoption and sales.
Telit Cinterion has over 30 years of knowledge and experience in the IoT space. Our secure, reliable technology and connectivity can help you meet evolving regulations and deliver top-quality patient care.
NExT connected modules are an easy-to-use solution for optimizing RTM. For less than a dollar a month, you get all the benefits to fast track your RTM devices to market.
Speak with our IoT experts to explore NExT connected modules for your connected health care solution.